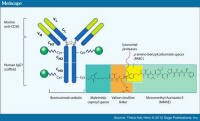
 An antibody-drug conjugate is a man-made monoclonal antibody* with a toxin linked to it. In this way the antibody becomes a way to deliver the toxin in a more targeted way - only to cells to which the antibody sticks - in order to increase its efficacy and reduce the risk. An antibody-drug conjugate is a man-made monoclonal antibody* with a toxin linked to it. In this way the antibody becomes a way to deliver the toxin in a more targeted way - only to cells to which the antibody sticks - in order to increase its efficacy and reduce the risk.
* What is an antibody (or mono-clonal antibody)?
When your body detects something that does not belong, such as bacteria, one way it eliminates the threat is to produce antibodies that bind to the protein shapes that are unique to the pathogen.
See for a video explanation by Roche: 
Brentuximab vedotin (below) is the most well known of this class of agents,
but other antibody-drug conjugates are in clinical phase testing.
Antibody-drug conjugates in clinical phase testing
ADCT-301 – A Novel Antibody-drug Conjugate Against Lymphomas – Moves Into Phase I Clinical Trial ADC Review http://bit.ly/2sB0obj
|
|
|
SGN-CD19A
anti-CD19 + toxin
Find trials | Reports |
SAR3419
anti-CD19 + maytansinoi
Find trials | Reports |
DT2219ARL
anti-CD19 & 22 antibody + immunotoxin
Find trials | Reports |
Inotuzumab Ozogamicin
anti-CD22 + calicheamicin
Find trials | Reports |
DCDT2980S
anti-CD22, conjugated to MMAE
Find trials | Reports |
LMB-2 immunotoxin
anti-CD25 antibody + immunotoxin
Find trials | Reports |
Adcetris ®
(SGN-35 / brentuximab vedotin)
anti-cd30 + antitubulin
Find trials | Reports |
|
|
In the News and New Resources
* Therapeutic potential of SGN-CD19B, a PBD-based anti-CD19 drug conjugate, for treatment of B-cell malignancies http://bit.ly/2ja9h96
* Technical but readable background on this technology
.gif) |
ADCT-301 – A Novel Antibody-drug Conjugate Against Lymphomas – Moves Into Phase I Clinical Trial ADC Review http://bit.ly/2sB0obj
|
* Effects of antibody, drug and linker on
preclinical and clinical toxicities of ADCs
full text: Europe PMC http://bit.ly/2dvbu9p
* Denintuzumab Mafodotin
(SGN-CD19A antibody-drug conjugate)
With RCHOP/RCHP VS RCHOP in DLBC or FL Lymphoma http://bit.ly/2amcOPr
Report on Phase 1 safety: http://bit.ly/2aXcLdS
* ASCO Post:
Inotuzumab Ozogamicin (drug-antibody conjugate) Plus Low-Intensity Chemo: A Winner in Older Patients With Leukemia http://bit.ly/1wnZIGP
Comment: IO is drug bound to an antibody – a conjugate. The conjugated antibody binds to cd22 on normal and malignant b-cells. When it binds to cd22 the drug attached to the antibody is internalized into the cells leading to cell damage and apoptosis.
Of interest to me is the idea of combining this with lower doses of standard drugs to potentially reduce toxicity and improve efficacy.
The optimal value of an agent can be hard to estimate when tested as a single agent. As with Rituxan, the potential is also based on how readily it can be combined with other effective agents (without overlapping toxicity and with a mechanism that is complementary and not redundant). In this example, IO given with lower doses of chemotherapy agents improved efficacy and reduced toxicity. What we want and need!
PAL query:
inotuzumab ozogamicin trials for lymphoma http://bit.ly/8Bzg5Z
Measuring our impact and interest in this agent:
so far there have been 216 clicks on this query. See https://bitly.com/8Bzg5Z+ )
*Onclive 2014:
Experts discuss Novel ADCs in Development for Lymphoid Malignancies http://bit.ly/1k9Bk51
* Haematologica 2013
Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. http://1.usa.gov/1hwm6Sz
* MNT 2013:
Patients with non-Hodgkin lymphomas resistant to treatment benefit from drug-antibody pair http://bit.ly/18IETHK
* Brentuximab Vedotin Shows Activity in B-Cell Lymphomas http://bit.ly/1c2gHTD
* Medical Xpress 2013:
Drug-antibody pair has promising activity in non-Hodgkin lymphoma http://bit.ly/1e3Jlp3
* ASH Paper, 2013: Phase 1/2 Study Of Brentuximab Vedotin In Pediatric Patients With Relapsed Or Refractory (R/R) Hodgkin Lymphoma (HL) Or Systemic Anaplastic Large-Cell Lymphoma (sALCL): Preliminary Phase 2 Data For Brentuximab Vedotin 1.8 Mg/Kg In The HL Study Arm http://bit.ly/1axwojx
* Paper: FDG-PET Adapted Sequential Therapy With Brentuximab Vedotin and Augmented ICE Followed By Autologous Stem Cell Transplant For Relapsed and Refractory Hodgkin Lymphoma http://bit.ly/1da0FUc
* ASH Paper: [Rare] Pancreatitis In Patients Treated With Brentuximab Vedotin: A Previously Unrecognized Serious Adverse Event http://bit.ly/1evaFJA
* Dana Farber 2013:
Drug-antibody pair has promising activity in non-Hodgkin lymphoma http://bit.ly/18RLL8n
Brentuximab Vedotin (Adcetris)
Brentuximab vedotin has been approved for treatment of relapsed or refractory Hodgkin lymphoma and anaplastic T cell lymphoma. The trade name is Adcetris.
Adcetris is a antibody-drug conjugate that binds to a molecule called cd30 on cells in Hodgkin lymphoma and anaplastic T cell lymphoma - but also other types of lymphoma at different frequencies or densities.
This targeted antibody is linked to a potent toxin, which is released inside the cell the antibody sticks to.
Resources
.gif) |
Full prescribing information (Label): accessdata.fda.gov/
|
.gif) |
Brentuximab Vedotin Shows Activity in B-Cell Lymphomas http://bit.ly/1c2gHTD
|
|