NEWS ITEM:
A Surprising Match: Cancer Immunotherapy and Mismatch Repair | NIH Director's Blog http://1.usa.gov/1BH8mCG
snip: ... it supports the hypothesis that immunotherapy may be most effective against tumors with many mutations. (In the new study, the tumor cells deficient in mismatch repair contained more than 20 times as many mutations, on average, than tumor cells proficient in mismatch repair.) The idea is that the greater the number of DNA glitches in a tumor cell, the more abnormal proteins it will produce—and the more abnormal proteins that are generated, the greater the odds that the body’s immune cells will regard the tumor cells as “foreign” and target them for destruction.
Blocking PD - an immune checkpoint receptor:
How it is thought to work in some cancers ... including at least some types of lymphoma
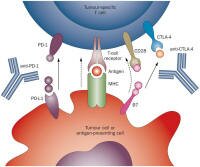 Anti-PD-1 antibody is an exciting new class of treatment. As illustrated in the graphic from Nature.com, the antibody blocks a signal used by some kinds of tumors that can suppress the immune system. By blocking the signaling between PD-1 and PD-L, the antibody can restore the cancer-fighting ability of t-cells. Anti-PD-1 antibody is an exciting new class of treatment. As illustrated in the graphic from Nature.com, the antibody blocks a signal used by some kinds of tumors that can suppress the immune system. By blocking the signaling between PD-1 and PD-L, the antibody can restore the cancer-fighting ability of t-cells.
Studies testing PD-1 antibody have reported clinical responses in relapsed and refractory Hodgkins lymphoma, leading the FDA to designate the PD-1 antibody as a breakthrough therapy for this indication. Clinical trials are under way to test the activity of PD-1 antibody in other types of lymphoma (see further below).
EXPERT OPINION:
Rather than targeting the tumor directly, immunotherapies inhibiting PD-1 pathway signaling modulate the antitumor immune response. Indeed, these agents have already demonstrated promising antitumor activity and manageable toxicity in various cancers. If future data continue to support encouraging clinical profiles of anti-PD-1 and anti-programmed death-ligand 1 antibodies, the current paradigm of cancer therapy may shift. In select patient populations (ideally identified by a predictive biomarker), PD-1 pathway-targeted immunotherapy has the potential to serve as the backbone of cancer treatment, and trials evaluating combinations with chemotherapy and/or molecularly targeted therapies will determine whether additive or even synergistic responses can be achieved.
Anti-programmed death-1 and anti-programmed death-ligand 1 antibodi... - PubMed - NCBI http://1.usa.gov/1sigZyH
Presently there are two commercial versions of the PD-1 antibody described on the NCI Drug Dictionary:
Urelumab (ue rel' ue mab; also known as BMS-663513 and anti-4-1BB antibody)
A humanized agonistic monoclonal antibody targeting the CD137 receptor with potential immunostimulatory and antineoplastic activities. Urelumab specifically binds to and activates CD137-expressing immune cells, stimulating an immune response, in particular a cytotoxic T cell response, against tumor cells. CD137 is a member of the tumor necrosis factor (TNF)/nerve growth factor (NGF) family of receptors and is expressed by activated T- and B-lymphocytes and monocytes; its ligand has been found to play an important role in the regulation of immune responses. Check for active clinical trials or closed clinical trials using this agent.
Nivolumab (nye vol' ue mab), nivolumab
A fully human monoclonal antibody directed against the negative immunoregulatory human cell surface receptor PD-1 (programmed death-1 or programmed cell death-1/PCD-1) with immunopotentiation activity. Nivolumab binds to and blocks the activation of PD-1, an Ig superfamily transmembrane protein, by its ligands PD-L1 and PD-L2, resulting in the activation of T-cells and cell-mediated immune responses against tumor cells or pathogens.
Activated PD-1 negatively regulates T-cell activation and effector function through the suppression of P13k/Akt pathway activation.
Tumor cells can up-regulate PD-L1 in order to “turn off” T cells that might destroy them.
The center panel illustrates PD-1 / L interaction leading to exhausted t-cells

Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3892798/figure/fig01/
Background
* UCLA's Dr. Antoni Ribas Discusses PD1 and PD-L1 Targeted therapy
Video on this promising new approach to treating the immune system: youtube.com/
* NEJM: Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer
The approach of blocking the PD-1 receptor with an antibody “induces very durable responses in patients who are otherwise treatment-refractory, which is a remarkable feature, and while these are very preliminary data they give us an important lead for further investigations.” (Topalian)
Immune modulating agents in clinical phase testing
|
Anti-PD-1 antibody
Find all PD-1 Lymphoma and CLL clinical trials
See Reports on this antibody
|
ipilimumab anti-CTL-A (MXD-010)
Find trials
Reports
|
Revlimid ® /
Lenalidomide / CC 5013 Find trials
Reports
|
* Mayo Clinic: Testing Anti-PD-1 Antibody (MK-3475) in Relapsed/Refractory CLL and Other Low Grade B Cell Non-Hodgkin Lymphoma http://1.usa.gov/1Kthf3R
|
|
|
* Testing Urelumab (IMID antibody) With Nivolumab (pd1 antibody) in Solid Tumors & B-cell Non-Hodgkins Lymphoma - http://1.usa.gov/ZwSKA7
Recruiting sites (none yet posted as of this writing) have contact information. Please contact the sites directly. If there is no contact information, you can email: |
|
|
Related Topics
Immune-based therapy
Lymphoma Simplified
Clinical trials by Type of Agent | Focus on Clinical Trials
News and Reports
.gif) |
PD-1 Blockade in Mediastinal Gray-Zone Lymphoma — NEJM http://bit.ly/2sIKvjc
"The high frequency of 9p24.1 copy-number alterations across mediastinal lymphomas suggests a disease-specific, genetically determined dependence on PD-1 for survival. These cases provide early evidence for using PD-1 inhibition in relapsed or refractory mediastinal gray-zone lymphoma, which warrants further testing."
|
.gif) |
New on Cancer Currents: Checking in on Cancer Checkpoint Inhibitors http://1.usa.gov/1OoFtuB
Excellent explanations. However, it does not cover breakthrough status of pd-1 antibody in Hodgkin's disease
|
.gif) |
PD1 ligand associated with poor overall survival in patients with diffuse large B-cell #lymphoma. -
PubMed - NCBI http://1.usa.gov/1gmMumj
|
.gif) |
Focus on: Tumour immunology & immunotherapy
The blockade of immune checkpoints in cancer immunotherapy http://bit.ly/Xc6Z4E
Drew M. Pardoll
|
.gif) |
NEJM: Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer
http://www.nejm.org/doi/full/10.1056/NEJMoa1200690
|
.gif) |
Yale J Biol Med > v.84(4); Dec 2011: Blockade of the B7-H1/PD-1 Pathway for Cancer Immunotherapy http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3238327/
|
.gif) |
Yale J Biol Med > v.84(4); Dec 2011: Blockade of the B7-H1/PD-1 Pathway for Cancer Immunotherapy
|
.gif) |
AACRL Phase I Safety and Pharmacokinetic Study of CT-011, a Humanized Antibody Interacting with PD-1, in Patients with Advanced Hematologic Malignancies http://clincancerres.aacrjournals.org/content/14/10/3044.long
|
.gif) |
JEM: Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation jem.rupress.org
PD-1 is an immunoinhibitory receptor expressed by activated T cells, B cells, and myeloid cells.
The relative levels of inhibitory PD-L1 and costimulatory B7-1/B7-2 signals on antigen-presenting cells may determine the extent of T cell activation and consequently the threshold between tolerance and autoimmunity.
PD-L1 expression on nonlymphoid tissues and its potential interaction with PD-1 may subsequently determine the extent of immune responses at sites of inflammation.
|
.gif) |
FDA Approves Yervoy (Ipilimumab, Anti-Ctla-4), An Immunotherapy, For Treatment Of Metastatic Melanoma - First-in-class Antibody Drug May Also Significantly Improve Standard Cancer Treatments
|
.gif) |
ASCO Post 2012: PD-1 Immune Checkpoint Inhibitors Look Promising in Multiple Solid Tumors ascopost.com
"But the newsmakers at ASCO were inhibitors of the PD-1 checkpoint.
The anti–PD-1 (BMS-936558) and anti-PD-L1 (BMS-936559) antibodies potentiate immune responses by blocking the interaction between the PD-1 protein, a T-cell co-inhibitory receptor, and one of its ligands, PD-L1—critical players in the ability of tumor cells to evade the host’s immune system."
“Ipilimumab and the anti–PD-1 and PD-L1 antibodies are all immune checkpoint drugs, but they are distinct in how they act,” Dr. Topalian explained at the press briefing. “The anti-CTLA4 agent is more global, whereas the others are more tissue-specific, so the toxicity is a bit less with them.”
|
* Investigational PD-1 Immune Checkpoint Inhibitor Nivolumab Receives U.S. FDA Breakthrough Therapy Designation for Hodgkin Lymphoma http://bit.ly/1rE0OL6
* Sponsor background:
Discover the role of the PD-1 pathway in cancer http://bit.ly/1h9BAxl
* ASCO Post 2013:
Anti–PD-1 Antibody Pidilizumab Plus Rituximab Shows High Activity in Relapsed Follicular Lymphoma http://bit.ly/19hCuqg
* BMT 2014:
“Allogeneic hematopoietic cell transplantation for diffuse large B cell lymphoma: who, when and how?” http://bit.ly/1eyiWiF
* OncLive:
Anti-PD-1 Antibody Pidilizumab Shows Promise in Diffuse Large B-Cell Lymphoma http://bit.ly/1l7DPow
* ASH 2012: Phase II Safety and Efficacy Study of CT-011, a Humanized Anti-PD-1 Monoclonal Antibody, in Combination with Rituximab in Patients with Relapsed Follicular Lymphoma http://bit.ly/Rl5EYX*
* The Lancet 2013:
Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial http://bit.ly/1fM2nxH
* Science Daily 2013:
Targeted Antibody, Immune Checkpoint Blocker Rein in Follicular Lymphoma http://bit.ly/1gwQKNS
* Bionnews Texas 2013:
Targeted Antibody, Immune Checkpoint Blocker Combo Safe, Effective Follicular Lymphoma Treatment http://bit.ly/1bL4Mbb
* Med xpress 2013
Targeted antibody, immune checkpoint blocker rein in follicular lymphoma (pidilizumab and Rituxan) http://bit.ly/JfFTKz
One drug attacks tumor cells directly, the other treats the immune system by taking the brakes off T cell response. Together, they put half of the patients with relapsed follicular lymphoma into complete remission in a phase II clinical trial at The University of Texas MD Anderson Cancer Center.
* Med xpress 2013
Targeted antibody, immune checkpoint blocker rein in follicular lymphoma (pidilizumab and Rituxan) http://bit.ly/JfFTKz
One drug attacks tumor cells directly, the other treats the immune system by taking the brakes off T cell response. Together, they put half of the patients with relapsed follicular lymphoma into complete remission in a phase II clinical trial at The University of Texas MD Anderson Cancer Center.
* ASH 2012: Phase II Safety and Efficacy Study of CT-011, a Humanized Anti-PD-1 Monoclonal Antibody, in Combination with Rituximab in Patients with Relapsed Follicular Lymphoma http://bit.ly/Rl5EYX
|