For sharing this topic: * An advocate’s perspective: Future value of Radioimmunotherapy
-- rationale for continued study of protocols based on RIT given with curative intent for indolent lymphoma, with shorter duration of treatment and costs http://bit.ly/1TSS9Df
ASCO Post: Limited Access to Radioimmunotherapy in Community Setting
May Lead to Extinction of a Unique Lymphoma Treatment http://bit.ly/2cOp48Z
In a review article "Speed Bumps on the Road to A Chemotherapy-Free World for Lymphoma Patients"
Bruce D. Cheson, M.D. writes:
“With the increasing number of targeted agents for the treatment of patients with lymphoid malignancies comes the promise of safe and effective chemotherapy free treatment strategies. A number of single agents, such as ibrutinib and idelalisib, have demonstrated impressive efficacy with a favorable toxicity profile.
The observations that most responses are, however, partial and treatment duration is indefinite, have stimulated interest in combinations of these agents with chemotherapy as well as with each other. Despite the promise of this approach, several recent trials of combinations of agents have been terminated as the result of life-threatening and fatal complications. Such outcomes have generated a cautionary note of the potential for unforeseen adverse effects that challenge drug development and mitigate against the empiric combination of such drugs outside of a clinical trial setting.”
While RIT was not discussed in this otherwise excellent article, I expect that Dr. Cheson recognizes its potential. Apparently, he does not believe that RIT can overcome the access issue in the community setting – that oncologists will not take the time to describe the risk/benefit profile of a treatment that requires that they send their patients elsewhere to receive it – particularly when there are often reasonable alternatives to be given directly by the community oncologist.
My fear is this: continuing low usage of RIT could lead to its discontinuation in the very near future. I cannot yet accept this as fate due to its unique properties and proven efficacy. I believe Dr. Cheson’s article on the challenges of testing targeted agents strengthens the case for additional testing of RIT (not Rituxan) doublets with the intent of curing more patients with indolent lymphoma in the near future – this with less physical and financial toxicity – and with substantially less cost, burden, and time on treatment.
Catch 22: “Most of the doctors who see and treat patients with NHL are not allowed to inject Zevalin. And those who are authorized to inject Zevalin, the nuclear medicine doctors, they don't see patients with non-Hodgkin’s lymphoma.
The unique features of RIT:
- It takes about 1 week to give the entire treatment, (minutes for each infusion) compared to many months of chemotherapy.
- It's the only non-chemotherapy-based approach with a very high rate of durable remissions.
Larson, Press: Radioimmunotherapy of human tumours - Europe PMC Article - Europe PMC http://bit.ly/1TXvYZt
“Seven Phase II studies and two Phase III studies have tested RIT in patients newly diagnosed with NHL who received front-line therapy either alone or as consolidation following chemotherapy.3, 60–67
These studies have all demonstrated efficacy with ORRs of 90–100% and CRs of 60–100% (Figure 3A). Also, the CRs induced by this approach have been very durable, with median remission durations exceeding six years in many studies.”
Review: 90 Y-ibritumomab tiuxetan (Zevalin radioimmunotherapy):
a nearly forgotten opportunity http://bit.ly/29snERA
Patrizia Mondello, Salvatore Cuzzocrea, [...], and Michael Mian
It's an important and unique choice for patients:
- who must continue to work through or shortly after treatment
- who cannot tolerate chemotherapy, because of advanced age, or specific comorbidities
- who may prefer to limit the on-treatment side effects specific to chemotherapy such as nausea, neuropathy, hair loss, gastric, and gastric and mucositis complications.
The future value of RIT could be far greater than we realize and could well address the challenges and findings cited by Dr. Cheson above – particularly if utilized early in the disease course. We ought not let enthusiasm for targeted drugs obscure the proven efficacy and potential value of radioimmunotherapy for the following reasons:
1) Antibody-based therapies have fewer anticipated adverse drug-interactions
Unlike chemotherapy agents and targeted drugs, antibodies are proteins that are normally produced by the body to fight infection. Unlike most classes of drugs, antibodies do not stress the primary organs that remove toxins: such as the liver and kidneys. Like these (and like Rituxan), RIT can be combined with other drugs with fewer anticipated adverse drug-to-drug interactions.
There are many promising RIT Doublets and Triplets to test. Having as the goal to enhance RIT, the targeted agents may also be given for shorter time periods – the study schedule based on the Pharmacokinetic properties of RIT. Such schedules could reduce the toxicity that comes with indefinite or long term use of targeted agents including maintenance Rituxan.
- Pre-targeting antibodies to clear normal b-cells before RIT seems a promising way to further increase the potency of RIT. (non-competing c19 antibody instead of cd20, for example, so that the radio-labeled cd20 does not have to compete with non-labeled monoclonal antibody for the same receptor)
- Immune modulating agents, such as Lenalidomide, or PD1/L antibodies seems a promising way to complement the immunotherapy-based mechanisms of action of RIT.
Radioimmunotherapy with Anti-CD20 Antibody May Trigger Long-Term Protective T Cell Immunity in Follicular Lymphoma http://1.usa.gov/1xRw4EY
- Drugs targeting cellular pathways, such as Ibrutinib, Selinexor, ABT-199 / Venetoclax may enhance the direct killing effects of the radiation delivered by antibodies.
- Epigenetic agents may be given with or prior to RIT (priming the cells to be more sensitive to radiation) sequentially … instead of with RIT to further improve the safety.
- Radio sensitizing agents combined with RIT such as Velcade. As in this admittedly small study which reported an 85% CR rate for Follicular in the relapse setting. See Phase 1 study of radiosensitization using bortezomib relapsed lymphoma receiving radioimmunotherapy http://1.usa.gov/281AWvz
"Sixteen patients responded (64%), including 44% complete responses (CRs), with 82% CR in patients with follicular lymphoma (FL). At a median follow-up of 7 months, median progression-free survival was 7 months, and seven of 11 patients with FL remained in remission at a median of 22 months."
Other agents may come into the pipeline:
See for example: Preclinical evaluation of a prodrug for radiosensitization in mouse and human tissues. http://bit.ly/1svhxCR
and Poly(ADP-ribose) polymerase inhibitors (PARP inhibitors) such as ABT-888 combined with external beam and
radioimmunotherapy to treat aggressive lymphoma http://bit.ly/2bYNix2
3) Enhancing RIT’s immune-mediated activity
One way that RIT is thought to work is by activating the immune system to attack tumor cells. Because of the very short duration of RIT treatment and because its activity is focused on b-cells expressing cd20, the negative impact on effector cells (other types of immune cells) seems limited compared to repeated use of chemotherapy over many months. RIT’s negative impact on the b-cell compartment of the immune system is less than Rituxan due to the short duration of treatment (2 days), -- compared to Rituxan which is typically given every 3 months for 2 years. 2 years of maintenance leading to long term b-cell depletion … and not infrequently to a higher incidence of infections that can require IV infusions of Immunoglobulins (naturally occurring antibodies produced by mature b-cells).
Related article, 2016: Radiotherapy combination opportunities leveraging immunity for the next oncology practice - Herrera - 2016 - CA: A Cancer Journal for Clinicians - Wiley Online Library http://bit.ly/2dpAOUr
Describes
4) The targeted delivery of radiation by RIT offers a better potential to address tumor heterogeneity.
Lymphoma cells are known to be sensitive to radiation a mechanism of action that is not pathway-specific.
So-called precision medicine by targeted drugs is limited by tumor heterogeneity – that is the tumor cells have varying types of genetic changes and abnormal pathways that are active within the cells. Tumor heterogeneity exists within the same patient and in different patients with the same diagnosis. This limits the scope and efficacy of single drugs that target specific genetic changes.
5) How do the risks of RIT compare to standard therapies?
The most serious risks of RIT are equivalent to standard chemotherapy
While the radiation part of RIT adds risk (such as for MDS), this risk appears to be no greater than for standard chemotherapy (Kaminski) and this risk is very low in the first-line setting.
Czuczman, et al, Treatment-Related Myelodysplastic Syndrome and Acute Myelogenous Leukemia in Patients Treated With Ibritumomab Tiuxetan Radioimmunotherapy http://bit.ly/1Y2oNlN
6) The potential vaccine-like effects of RIT - inducing an immune response appears to be a key to achieving the most durable remissions
When tumor cells are killed by radiation, this may lead to a vaccine-like effect activating the immune system to attack tumor cells. This effect has been observed for local radiation where tumors in other parts of the body regress. (The abscopal effect). A vaccine effect for RIT is also suggested by the delayed time to optimal response to Bexxar RIT reported by Kaminski - well beyond the half life of the drug in the body.
"When given as initial treatment: "Responses were observed in 72 of the 76 patients, most of whom reported regression of palpable tumor within two weeks. Complete responses were observed in 57 of 76 patients (Table 1), with a median time to an evaluated complete response of 202 days (range, 55 to 693).
Also see Radiation therapy and cancer vaccines: Timing is everything http://bit.ly/1XM9Cg2
“In situ” vaccination for systemic effects in follicular lymphoma - Europe PMC Article - Europe PMC http://bit.ly/1RMIsym
6) Financial toxicity and sustainability challenges -- RIT based therapy can substantially reduce on-treatment time and related risks and costs
Decreasing on-treatment time is an important objective for clinical research … for the patient and for the health care system overall. When feasible, patients will be better served by a single course of treatment that achieves very high rates of durable remissions and cures. Being free of the disease and of treatment seems the ideal objective of clinical research … where this outcome is feasible. RIT doublets and triplets (given concurrently and/or in sequence) within weeks appear to have a unique potential to achieve this ideal for indolent lymphoma and deserves testing by the NCTN (NCI cooperative groups).
~ Perspective by Karl Schwartz,
Research advocate, President of PAL
With no financial conflict of interest to report
See also our letter educating our Senators about the impact of a burdensome training requirements for the administration of RIT on patient access to RIT in the community setting.
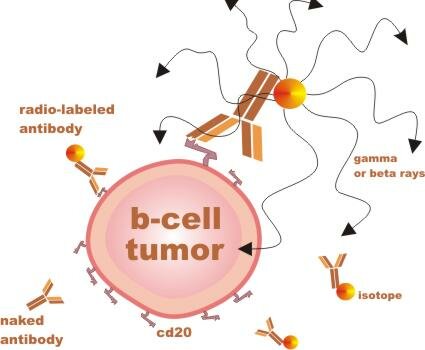 When your body detects something that does not belong, such as bacteria (pathogen), one way it eliminates the threat is to produce antibodies that bind to the protein shapes that are specific to the pathogen.
When your body detects something that does not belong, such as bacteria (pathogen), one way it eliminates the threat is to produce antibodies that bind to the protein shapes that are specific to the pathogen.
RIT agents are man-made antibodies with different radiation components attached. These antibodies are designed to bind to a protein shape called CD20, which sticks out of mature B lymphocytes (immune cells), both malignant and nonmalignant (cancerous and normal). ...
... Importantly, the cd20 shape (or antigen / receptor) is not found on precursor B cells - immature b-cells which can later mature to replenish the supply of normal mature b-cells.
RIT is considered a targeted therapy, because the antibodies that deliver the radiation are specific to one type of cell. RIT is more potent than unlabeled antibody therapy, such as Rituxan, but it also has unique potential risks.
RIT is given in therapeutic steps
(1) The initial antibody dose ("cold" or "naked" antibody) clears the body of normal b-cells so that subsequent doses will be more focused on tumor cells.
(2) The second "warm" dose has anti-tumor effects, but also helps calculate the optimal final dose based on uptake of the drug and individual clearance rates as determined by imaging of the gamma radioactive element.
(3) The final "hot" dose has has the most potent anti-tumor effects, and is focused on tumor cells, because prior doses have cleared the body of normal b-cells.
Possible mechanisms of action
When radio-labeled antibody binds to tumor cells it can cause tumor killing by
-
Apoptosis - programmed cell death triggered by the antibody
-
Complement-dependent cytotoxicity (CDC) - where antibody fixes complement that kills the tumor cells
-
Antibody-dependent cellular cytotoxicity (ADCC) - where effector cells (immune cells) kill the tumor cells
-
Ionizing radiation from the radioisotope damages the tumor cells, leading to cell death
-
Vaccine-like effect - leading to adaptive immunity against cells that may survive initial treatment